GHGH Formula - C14H26O11 - Over 100 million chemical compounds
Por um escritor misterioso
Last updated 04 junho 2024

GHGH contains total 51 atom(s); 26 Hydrogen atom(s), 14 Carbon atom(s), and 11 Oxygen atom(s). Learn more about GHGH chemical formula at Mol-Instincts.
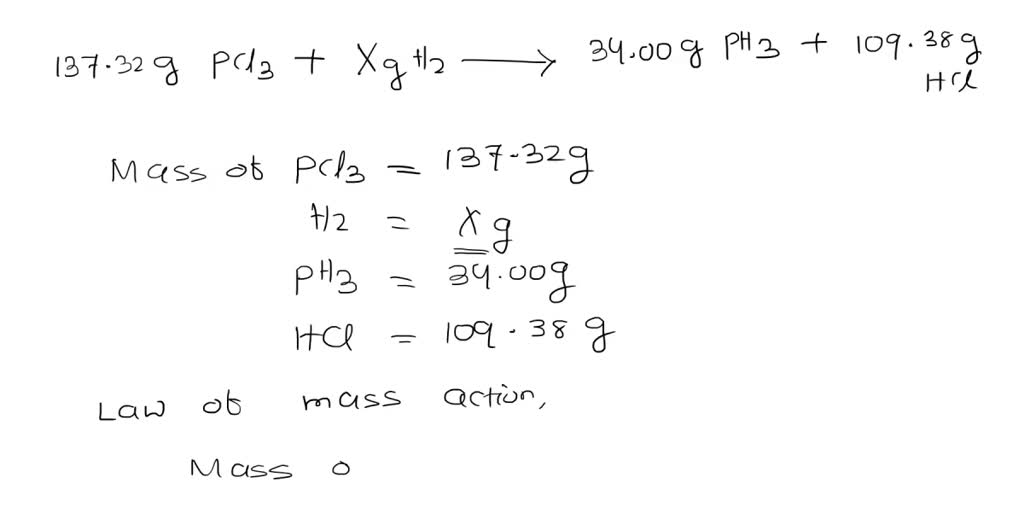
SOLVED: Determine the value of X in the following otherwise correct description of a chemical reaction: (137.32 g PCl3) + (X g H2) + (34.00 g PH3) = (109.38 g HCl) A
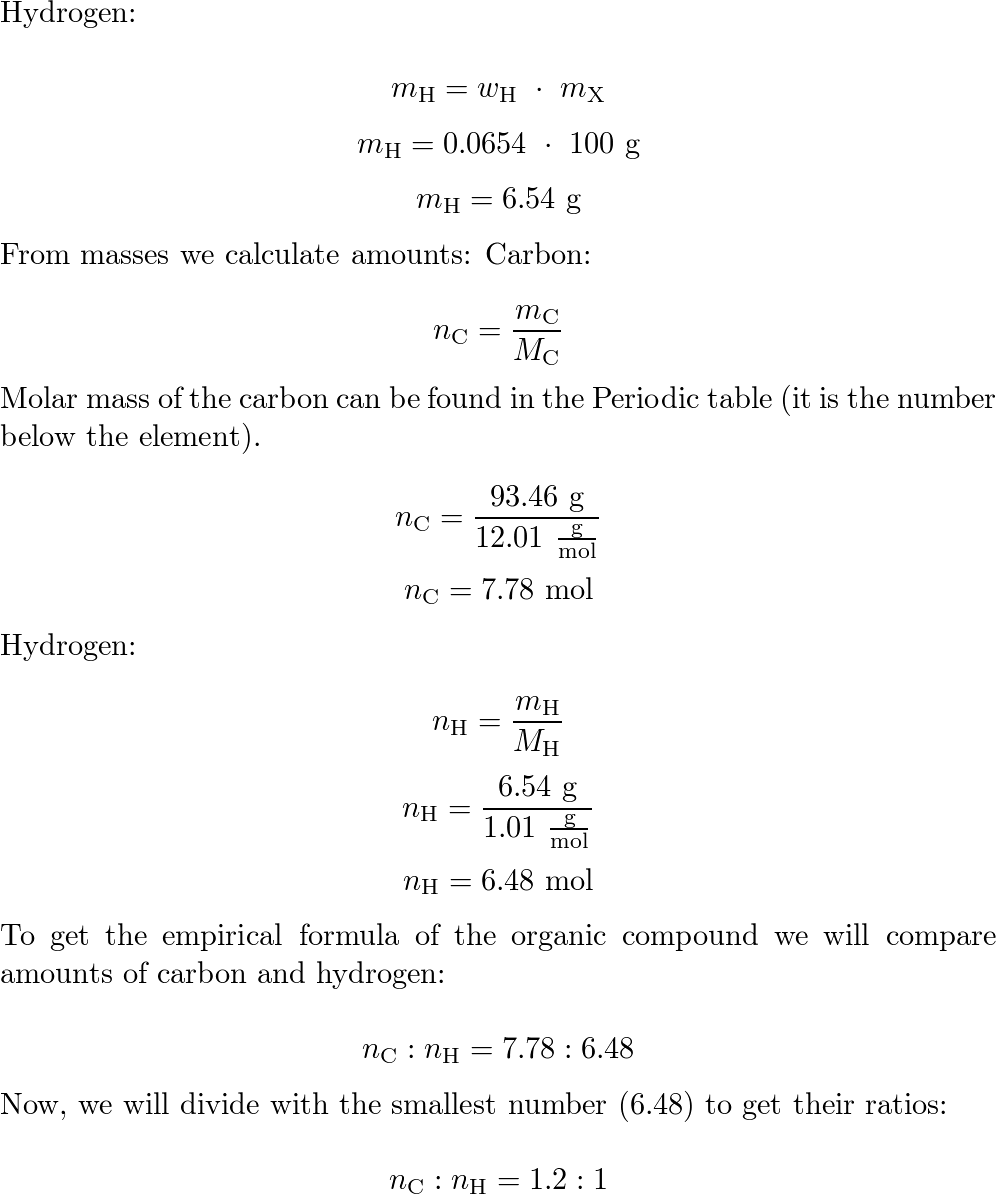

a 3178 b 6714 c 671 d 7 Question 46 Galactose is a compound with the following

A compound is found to contain 39.99% carbon, 6.727% hydrogen, and 53.28% oxygen by mass. The molar mass for this compound is 90.09 g/mol. What is the molecular formula for this compound?
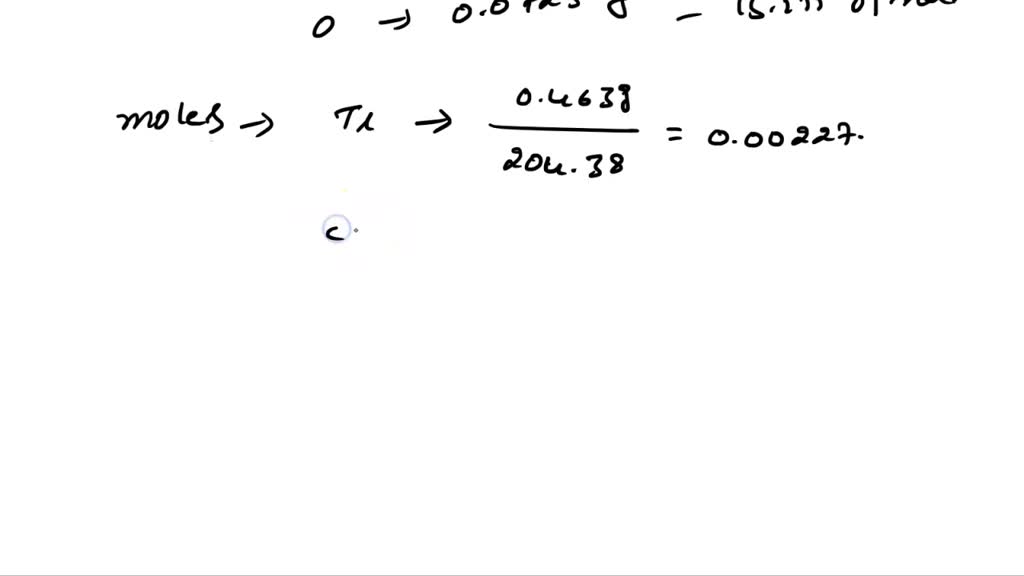
SOLVED: NAME the compound which contains 0.463 g Tl (#81) 0.0544 g of carbon, 0.00685 g of Hydrogen and 0.0725 g oxygen by finding its empirical formula: (1 pt) Draw a molecular

Solved 6.00 g of a certain Compound X, known to be made of
A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g
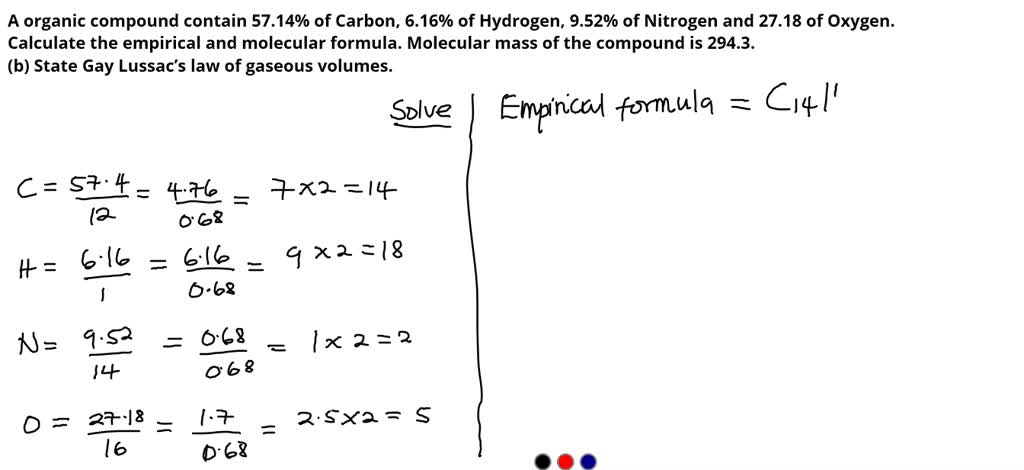
SOLVED: A organic compound contain 57.14% of Carbon, 6.16% of Hydrogen, 9.52% of Nitrogen and 27.18 of Oxygen. Calculate the empirical and molecular formula. Molecular mass of the compound is 294.3. -3-(b)
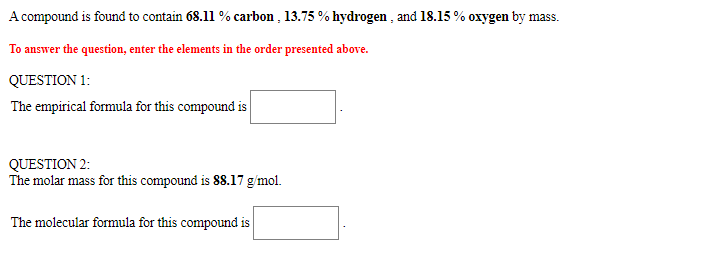
Solved A compound is found to contain 2.270 % hydrogen

Science Chapter 18! Study up! Flashcards
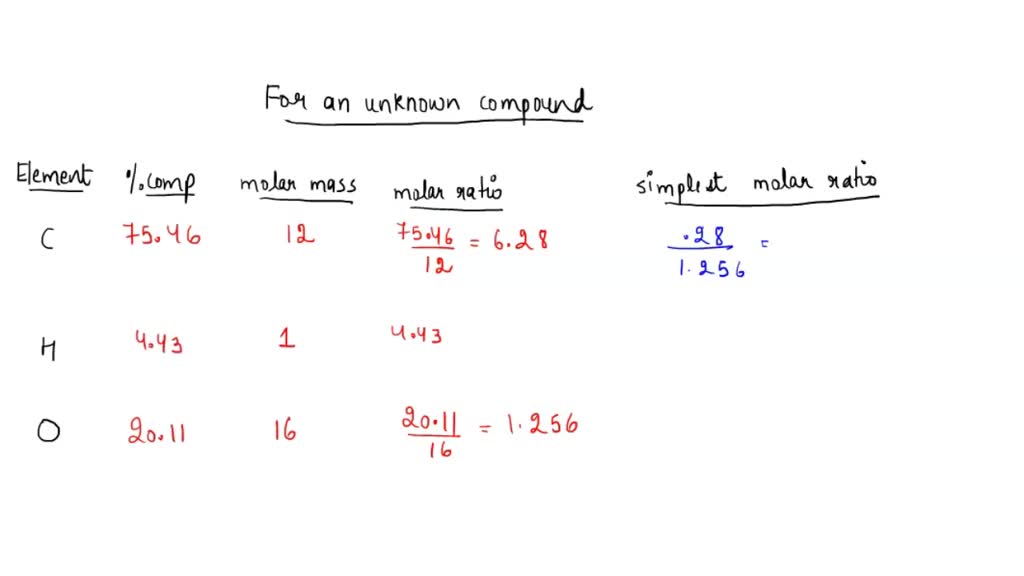
SOLVED: An unknown compound contains 75.46% Carbon, 4.43% Hydrogen, and 20.11% Oxygen by mass. The molecular mass is 318.31 g/mol. What is the molecular formula of the unknown compound?
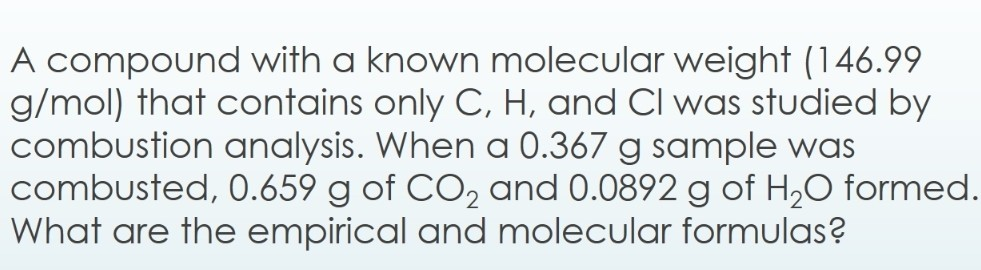
Solved A compound with a known molecular weight (146.99

SOLVED: 13.00 g of Compound X with molecular formula C4H6 are burned in constant-pressure calorimeter containing 30.00 kg of water at 25 %C The temperature of the water is observed to rise

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g
Recomendado para você
-
 Girls' Health in Girls' Hands A program for girls, by girls.04 junho 2024
Girls' Health in Girls' Hands A program for girls, by girls.04 junho 2024 -
 ghgh Thefamoushub04 junho 2024
ghgh Thefamoushub04 junho 2024 -
 ghgh view world round04 junho 2024
ghgh view world round04 junho 2024 -
 Unscramble GHGH - Unscrambled 0 words from letters in GHGH04 junho 2024
Unscramble GHGH - Unscrambled 0 words from letters in GHGH04 junho 2024 -
 Ghgh (kelaird) - Profile04 junho 2024
Ghgh (kelaird) - Profile04 junho 2024 -
Ghgh - song and lyrics by Chris Espo, Cortez04 junho 2024
-
rakka / ghgh piano fuzz04 junho 2024
-
 F-GHGH - Boeing 767-37EER, Air France04 junho 2024
F-GHGH - Boeing 767-37EER, Air France04 junho 2024 -
GuanHua Corporation (GHGH) Stock Message Board04 junho 2024
-
 ghgh gifs WiffleGif04 junho 2024
ghgh gifs WiffleGif04 junho 2024
você pode gostar
-
 Funko Pop Pokemon - Leafeon 86604 junho 2024
Funko Pop Pokemon - Leafeon 86604 junho 2024 -
 Vivian Lou Insolia® Classic Weight-Shifting Insoles for High04 junho 2024
Vivian Lou Insolia® Classic Weight-Shifting Insoles for High04 junho 2024 -
 ITEM GRÁTIS! COMO PEGAR o DOMINUS GRÁTIS DA PRIME GAMING! ROBLOX04 junho 2024
ITEM GRÁTIS! COMO PEGAR o DOMINUS GRÁTIS DA PRIME GAMING! ROBLOX04 junho 2024 -
 Significado de Impasse04 junho 2024
Significado de Impasse04 junho 2024 -
 PaperMonastery04 junho 2024
PaperMonastery04 junho 2024 -
Boba Bubble Tea: Doodling Game - Apps on Google Play04 junho 2024
-
 FNAF 1 Office - Five Nights At Freddy's by rocca, Download free STL model04 junho 2024
FNAF 1 Office - Five Nights At Freddy's by rocca, Download free STL model04 junho 2024 -
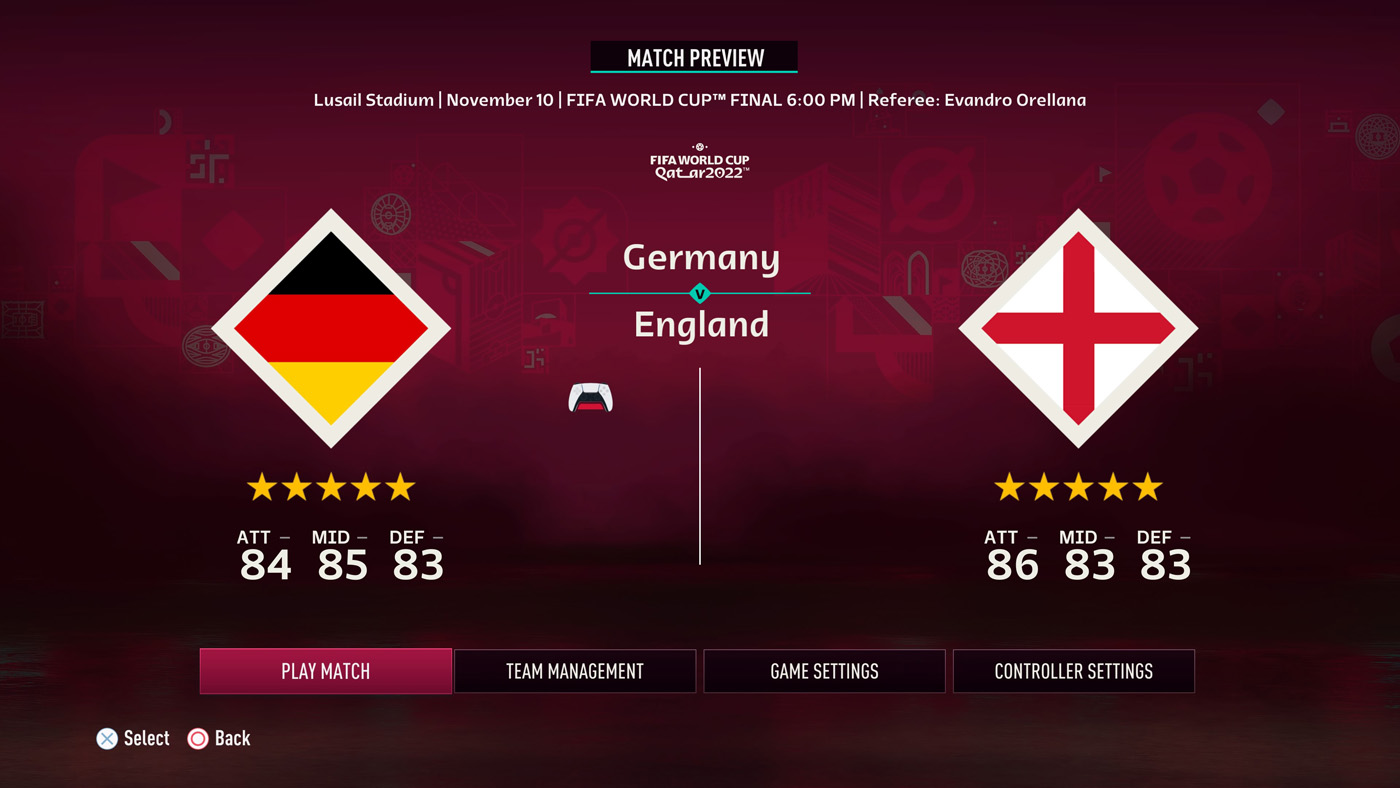 FIFA 23 – World Cup 2022 Kick Off – FIFPlay04 junho 2024
FIFA 23 – World Cup 2022 Kick Off – FIFPlay04 junho 2024 -
 Santos FC e American English Academy firmam parceria para o Sócio Rei, funcionários e atletas - Santos Futebol Clube04 junho 2024
Santos FC e American English Academy firmam parceria para o Sócio Rei, funcionários e atletas - Santos Futebol Clube04 junho 2024 -
 10 Explosive Speed Exercises No Equipment/Bodyweight Training04 junho 2024
10 Explosive Speed Exercises No Equipment/Bodyweight Training04 junho 2024

