Contributing to Evidence-Based Regulatory Decisions: A Comparison
Por um escritor misterioso
Last updated 18 junho 2024


The ABCs of Evidence-Informed Policymaking
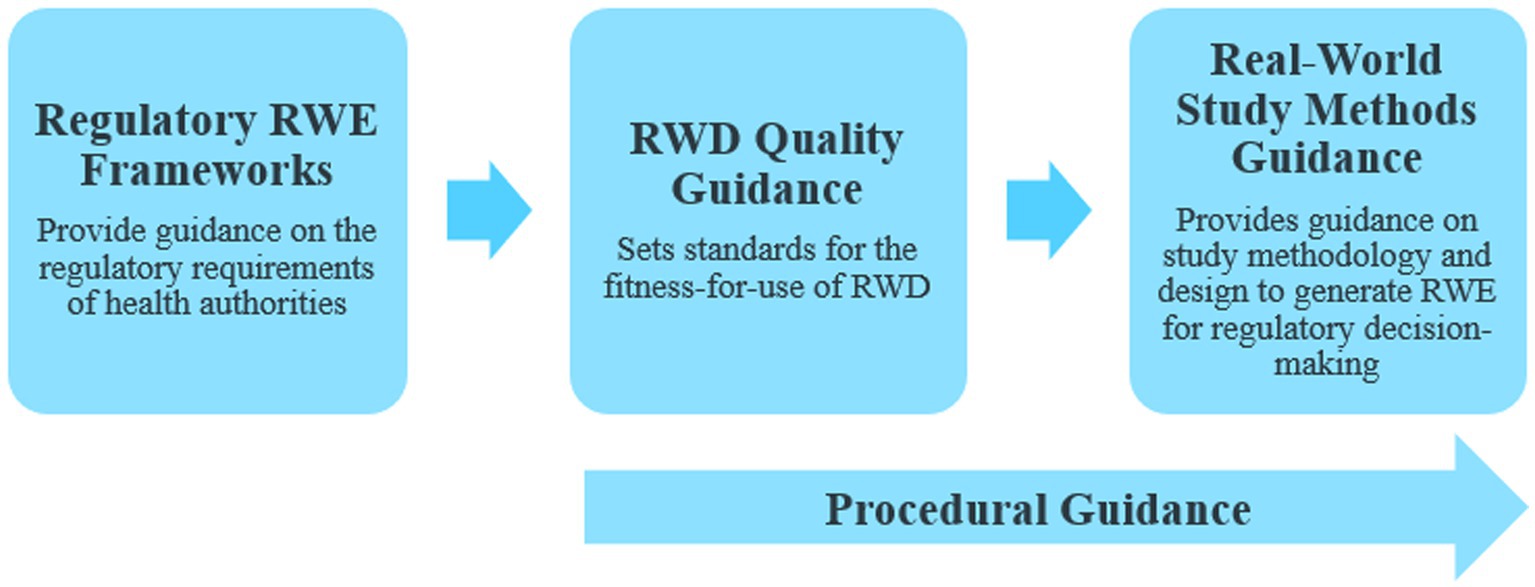
Frontiers Real-world evidence for regulatory decision-making: updated guidance from around the world

What Is Evidence-based Management? Evidence-based Management In A Nutshell - FourWeekMBA

Real-world evidence (RWE) in regulatory decision making: key use cases.

Proposed update to federal cost-benefit analysis guidelines correctly focuses on accounting for inequality in regulations - Equitable Growth
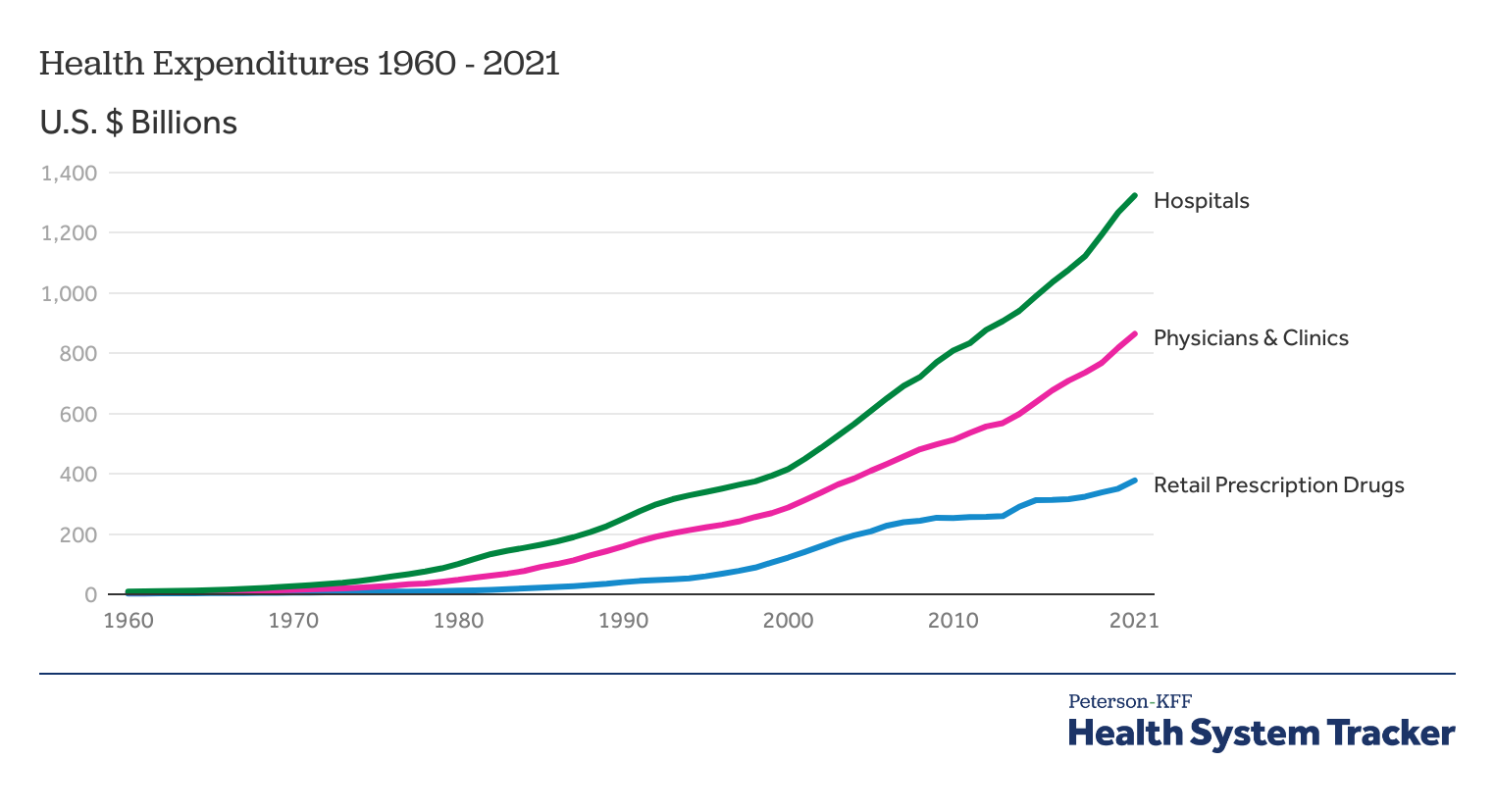
Nine reasons for rising healthcare costs
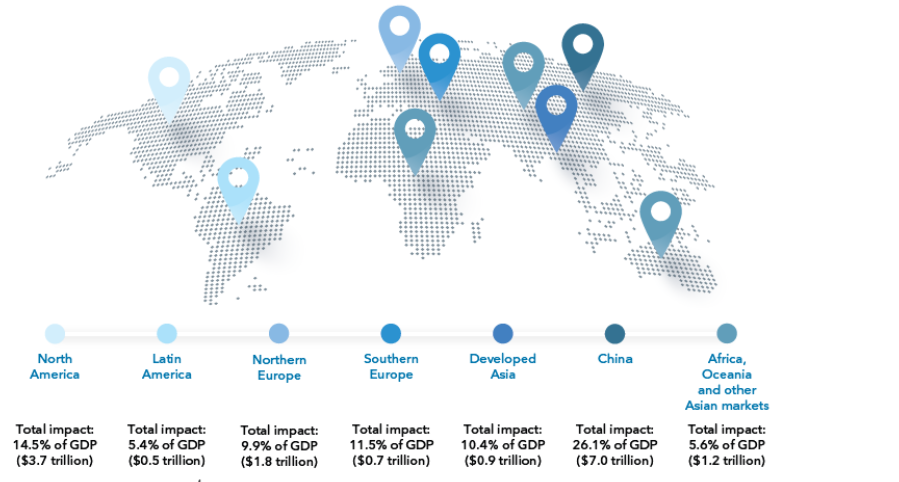
Transformative technologies (AI) challenges and principles of regulation

Organizational Evidence-Based and Promising Practices for Improving Clinician Well-Being - National Academy of Medicine

Necessity of strengthening the current clinical regulatory for companion diagnostics: An institutional comparison of the FDA, EMA, and MFDS: Molecular Therapy - Methods & Clinical Development
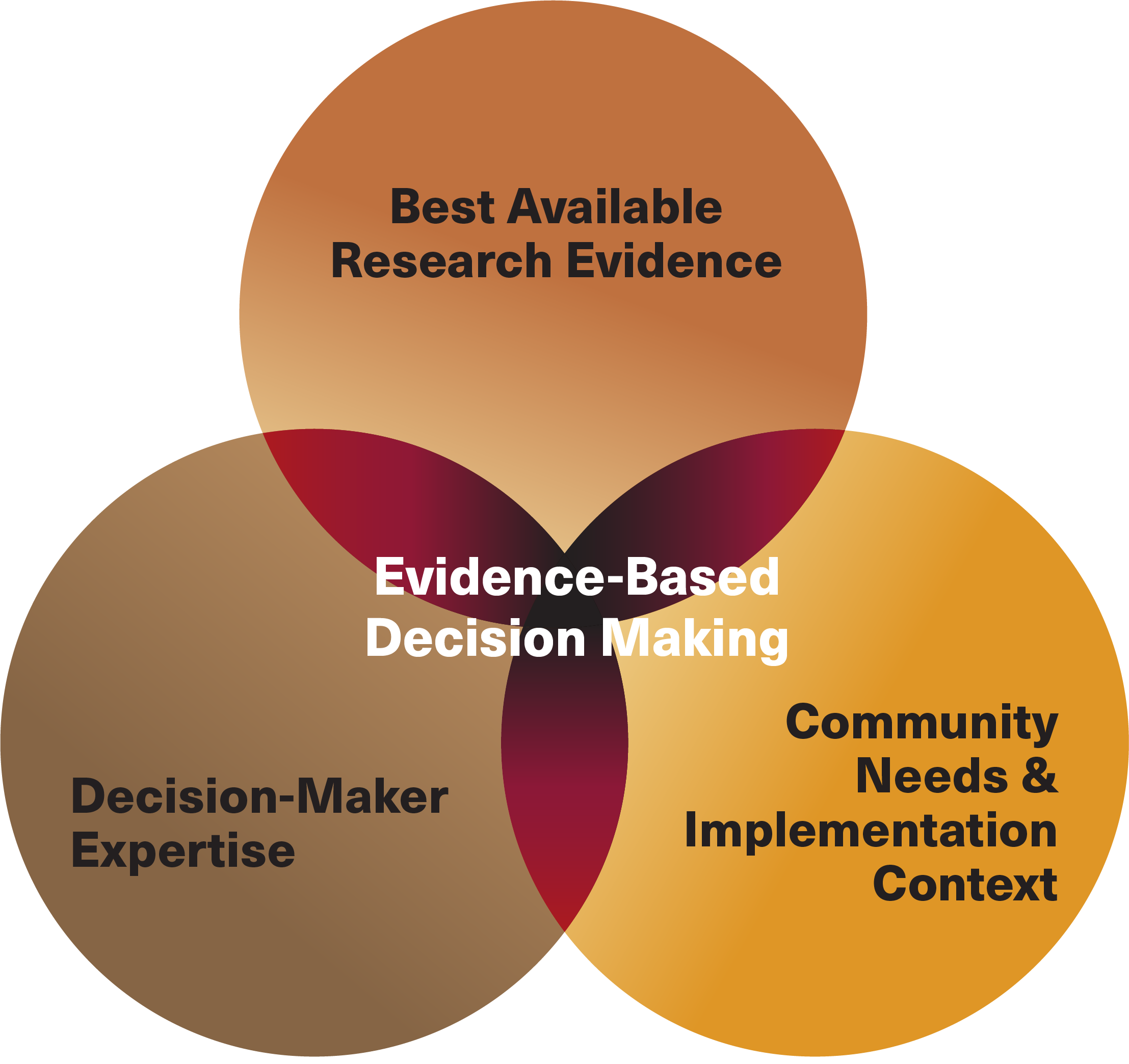
Evidence-Based Decision-Making in Policymaking: A 5-Year Vision for Colorado - Colorado Evaluation and Action Lab

NIH's Definition of a Clinical Trial
Recomendado para você
-
Shopee - If you wanna know what a flat clicker is, check Urban18 junho 2024
-
Urban Dictionary (@urbandictionary) • Instagram photos and videos18 junho 2024
-
 ProjektMelody 🥯 VSHOJO on X: I forgot you guys made me buy this18 junho 2024
ProjektMelody 🥯 VSHOJO on X: I forgot you guys made me buy this18 junho 2024 -
 Abbey road bag We Are Knitters18 junho 2024
Abbey road bag We Are Knitters18 junho 2024 -
 Royal Building of Mafra – Palace, Basilica, Convent, Cerco Garden18 junho 2024
Royal Building of Mafra – Palace, Basilica, Convent, Cerco Garden18 junho 2024 -
Cardinals coach Jonathan Gannon not in rush to get Kyler Murray18 junho 2024
-
 A Randomized Trial of a Multifactorial Strategy to Prevent Serious18 junho 2024
A Randomized Trial of a Multifactorial Strategy to Prevent Serious18 junho 2024 -
 Proceedings of the 2023 SIAM International Conference on Data18 junho 2024
Proceedings of the 2023 SIAM International Conference on Data18 junho 2024 -
Newfoundland and Labrador - Wikipedia18 junho 2024
-
NAE Website - Guru Madhavan18 junho 2024
você pode gostar
-
![The Medium [Gameplay] - IGN](https://assets1.ignimgs.com/2020/05/07/themedium-energyspot-logo-1588890590090.png) The Medium [Gameplay] - IGN18 junho 2024
The Medium [Gameplay] - IGN18 junho 2024 -
 Tokyo Ghoul - IGN18 junho 2024
Tokyo Ghoul - IGN18 junho 2024 -
 Fenrir, o pokémon lendário em uma aventura estilo desenho animado com fundo branco18 junho 2024
Fenrir, o pokémon lendário em uma aventura estilo desenho animado com fundo branco18 junho 2024 -
In this position, (black to move) Qe1 is apparently checkmate, but I am confused because the rook is pinned and therefore cannot defend the queen. Can someone explain this to me?18 junho 2024
-
 Kotoura-san – 07 – RABUJOI – An Anime Blog18 junho 2024
Kotoura-san – 07 – RABUJOI – An Anime Blog18 junho 2024 -
 Glen Hansard Live at Istropolis, Bratislava on 2019-11-17 : Free18 junho 2024
Glen Hansard Live at Istropolis, Bratislava on 2019-11-17 : Free18 junho 2024 -
 Shigeru Miyamoto Reveals Why Donkey Kong Got a Redesign For The18 junho 2024
Shigeru Miyamoto Reveals Why Donkey Kong Got a Redesign For The18 junho 2024 -
 Jogo Commando Attack no Jogos 36018 junho 2024
Jogo Commando Attack no Jogos 36018 junho 2024 -
 CHEATS - GTA SAN ANDREAS - CHEATS/CODIGOS/TRAPAÇAS XBOX-360 REMAKE18 junho 2024
CHEATS - GTA SAN ANDREAS - CHEATS/CODIGOS/TRAPAÇAS XBOX-360 REMAKE18 junho 2024 -
 Classroom of the Elite - Temporada 218 junho 2024
Classroom of the Elite - Temporada 218 junho 2024


