FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Last updated 02 junho 2024

CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.

Video: Depression is a Journey

FDA fast-tracks drug for dementia agitation that increases risk of death 4 times - Study Finds

FDA Approves Rexulti for Treating Agitation Due to Alzheimer's - MyChesCo
Video: Using the REXULTI Savings Card

Amgen Triumphs in $27.8 Billion Horizon Buyout Battle
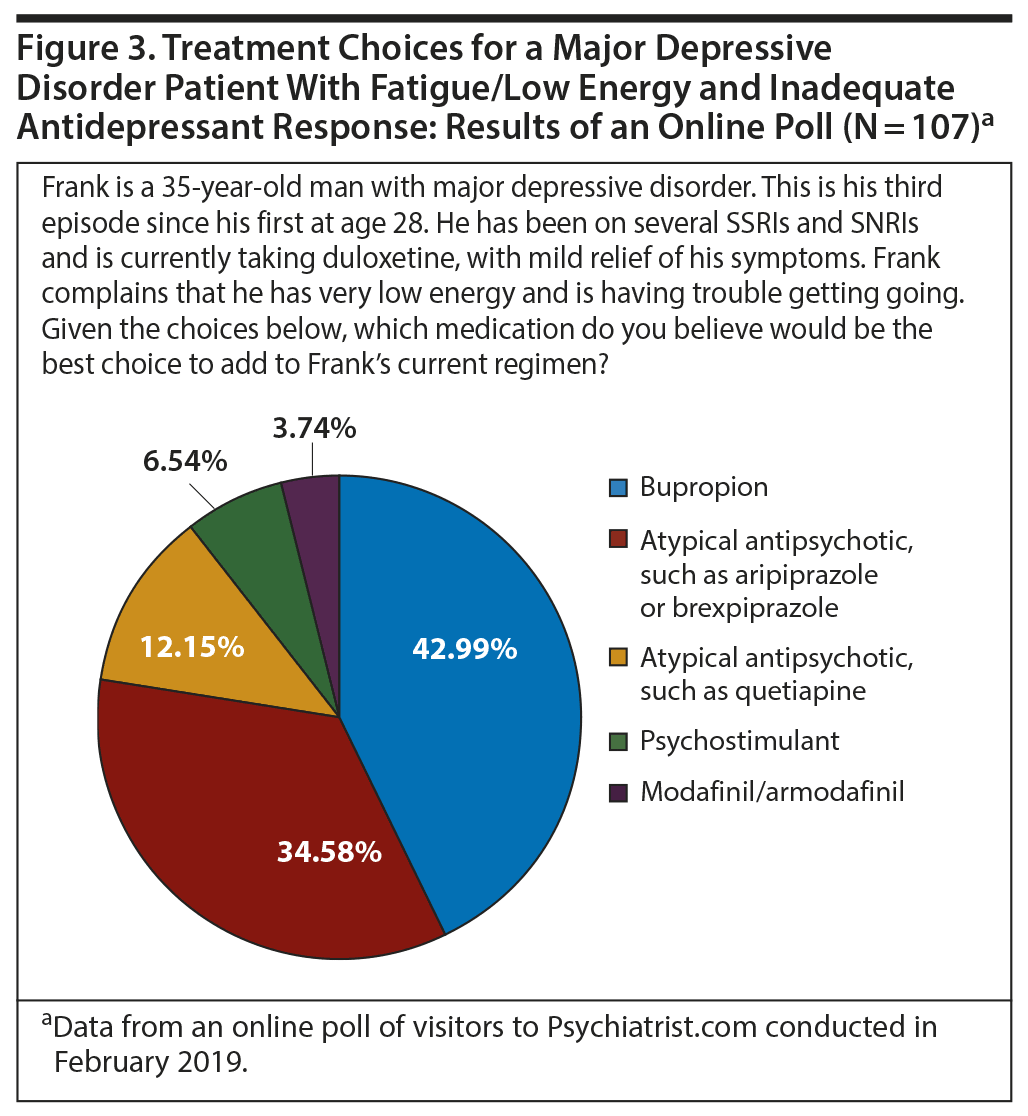
Activating and Sedating Properties of Medications Used for the Treatment of Major Depressive Disorder and Their Effect on Patient Functioning

Breakthrough Therapy Designation
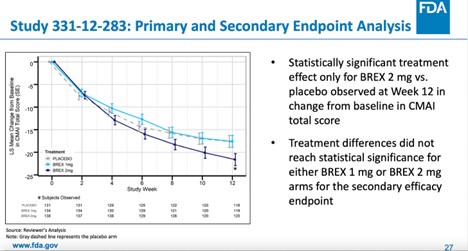
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

FDA rushes approval of dementia drug that quadruples risk of death

FDA approves supplemental new drug application for Rexulti to treat Alzheimer's agitation

FDA Approves Rexulti for Agitation Associated With Dementia Due to Alzheimer's Disease

FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services
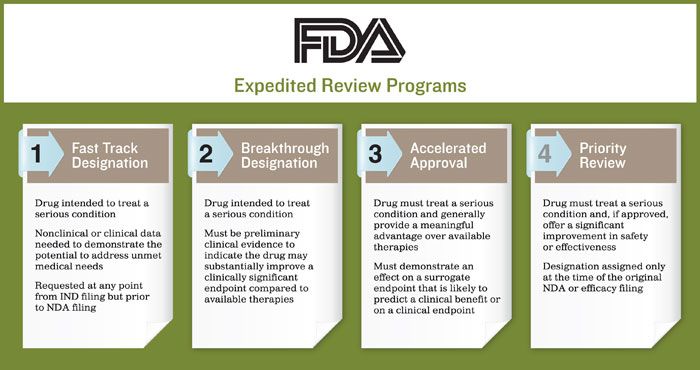
Accelerated Change: Understanding the FDA's Expedited Pathways
Recomendado para você
-
 Rexulti oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD02 junho 2024
Rexulti oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD02 junho 2024 -
Brexpiprazole (Rexulti): Uses, Side Effects, Warnings & More - GoodRx02 junho 2024
-
 Rexulti - Otsuka Pharmaceutical Co., Ltd.02 junho 2024
Rexulti - Otsuka Pharmaceutical Co., Ltd.02 junho 2024 -
 Rexulti 1mg Lundbeck 30 Comprimidos - Drogaria Sao Paulo02 junho 2024
Rexulti 1mg Lundbeck 30 Comprimidos - Drogaria Sao Paulo02 junho 2024 -
 Rexulti Filmtabl 1 mg 28 Stk auf Rezept kaufen02 junho 2024
Rexulti Filmtabl 1 mg 28 Stk auf Rezept kaufen02 junho 2024 -
 REXULTI (BREXPIPRAZOL) - NÃO TOME SEM ASSISTIR02 junho 2024
REXULTI (BREXPIPRAZOL) - NÃO TOME SEM ASSISTIR02 junho 2024 -
 Rexulti Advertisement Poster for Sale by BLTC02 junho 2024
Rexulti Advertisement Poster for Sale by BLTC02 junho 2024 -
 Pharmacy: Rexulti (Brand for Brexpiprazole, Oral Tablet)02 junho 2024
Pharmacy: Rexulti (Brand for Brexpiprazole, Oral Tablet)02 junho 2024 -
 REXULTI® (Brexpiprazole) FDA Approved for Treating Agitation in Alzheimer's Disease Patients02 junho 2024
REXULTI® (Brexpiprazole) FDA Approved for Treating Agitation in Alzheimer's Disease Patients02 junho 2024 -
 Net Present Value Model: Rexulti - Market Research Reports & Consulting02 junho 2024
Net Present Value Model: Rexulti - Market Research Reports & Consulting02 junho 2024
você pode gostar
-
Pokémon GO - Angra02 junho 2024
-
 Shredder Mini - Hand Cranked Plastic & Can Shredder02 junho 2024
Shredder Mini - Hand Cranked Plastic & Can Shredder02 junho 2024 -
 Super Tails! (Artist: Pearl_AG7777777) : r/SonicTheHedgehog02 junho 2024
Super Tails! (Artist: Pearl_AG7777777) : r/SonicTheHedgehog02 junho 2024 -
 JOGO E LIVRO DE COLORIR - PÁSCOA CRISTÃ02 junho 2024
JOGO E LIVRO DE COLORIR - PÁSCOA CRISTÃ02 junho 2024 -
 Underrated Roblox Games to Play When You're Bored02 junho 2024
Underrated Roblox Games to Play When You're Bored02 junho 2024 -
 Bayonetta 2 + Bayonetta Nintendo Switch02 junho 2024
Bayonetta 2 + Bayonetta Nintendo Switch02 junho 2024 -
The Outhouse Show02 junho 2024
-
 Goblin Slayer Side Story: Year One - The Fall 2018 Manga Guide - Anime News Network02 junho 2024
Goblin Slayer Side Story: Year One - The Fall 2018 Manga Guide - Anime News Network02 junho 2024 -
 SonAmy Wedding ❤️~ Sonic and Amy VS DeviantArt02 junho 2024
SonAmy Wedding ❤️~ Sonic and Amy VS DeviantArt02 junho 2024 -
 The Legend of the Legendary Heroes Tokyo International Anime Fair Trailer Available Online02 junho 2024
The Legend of the Legendary Heroes Tokyo International Anime Fair Trailer Available Online02 junho 2024

