CSPI asks FDA to ban powdered caffeine sold as a dietary supplement
Por um escritor misterioso
Last updated 12 junho 2024

The Center for Science in the Public Interest (CSPI) has sent a petition to federal regulators seeking the ban of pure, powdered caffeine that is packaged and sold as a dietary supplement. Because of the product’s extreme potency, the possibility of accidental overdose poses a clear and present public health risk, the organization asserts.

Potent Powdered Caffeine Raises Safety Worries : Shots - Health News : NPR

Laura MacCleery - Senior Director, Public Policy - UnidosUS (formerly National Council of La Raza)

Andrew F. Smith

FDA to explore effect of caffeine on the health of children
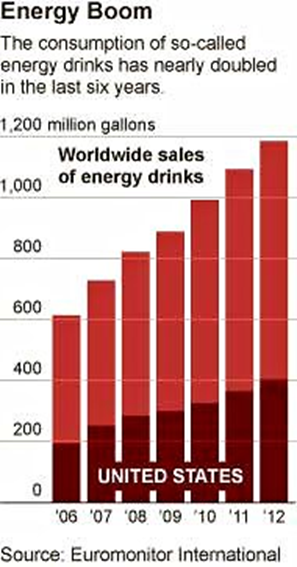
Caffeine Archives - Food Politics by Marion Nestle

FDA acts to pull concentrated caffeine from market

FDA Calls Powdered Caffeine Unsafe

FDA cracks down on illegal sales of pure caffeine

Does Prevagen Really Work?

User Clip: Dr. Robert Lustig thinks caffeine is not regulated
Recomendado para você
-
 BulkSupplements.com Whey Protein Concentrate Powder, 30g - Unflavored, Pure Protein Powder (100G - 3 Servings)12 junho 2024
BulkSupplements.com Whey Protein Concentrate Powder, 30g - Unflavored, Pure Protein Powder (100G - 3 Servings)12 junho 2024 -
 BulkSupplements Creatine Powder12 junho 2024
BulkSupplements Creatine Powder12 junho 2024 -
 Bulk Supplements Glutamine Review12 junho 2024
Bulk Supplements Glutamine Review12 junho 2024 -
Android Apps by BulkSupplements.com on Google Play12 junho 2024
-
 Bulk Supplements12 junho 2024
Bulk Supplements12 junho 2024 -
 BulkSupplements Review Failed Quality Tests and Great Prices – Illuminate Labs12 junho 2024
BulkSupplements Review Failed Quality Tests and Great Prices – Illuminate Labs12 junho 2024 -
 BulkSupplements.com Reviews - 19 Reviews of Bulksupplements.com12 junho 2024
BulkSupplements.com Reviews - 19 Reviews of Bulksupplements.com12 junho 2024 -
 Bulk Supplement Manufacturer and Supplier12 junho 2024
Bulk Supplement Manufacturer and Supplier12 junho 2024 -
 Health, Fitness, Nutrition and Supplement Reviews for You12 junho 2024
Health, Fitness, Nutrition and Supplement Reviews for You12 junho 2024 -
 BULK SUPPLEMENTS REVIEW! + What Supplements To Take To Get Bigger And Stronger Faster!12 junho 2024
BULK SUPPLEMENTS REVIEW! + What Supplements To Take To Get Bigger And Stronger Faster!12 junho 2024
você pode gostar
-
Isekai Cheat Magician Turning Point - Watch on Crunchyroll12 junho 2024
-
 Pokemon Mega Emerald X & Y - Play Game Online12 junho 2024
Pokemon Mega Emerald X & Y - Play Game Online12 junho 2024 -
 Pokemon Sun and Moon Review - Alola Baby! - The Escapist12 junho 2024
Pokemon Sun and Moon Review - Alola Baby! - The Escapist12 junho 2024 -
 Bothobot 2 Fighters Play Now Online for Free12 junho 2024
Bothobot 2 Fighters Play Now Online for Free12 junho 2024 -
/i.s3.glbimg.com/v1/AUTH_bc8228b6673f488aa253bbcb03c80ec5/internal_photos/bs/2022/x/Y/t2HsVhTdApuVf1zYwFFQ/gettyimages-1371370038.jpg) Juventus sai na frente em derby de Turim, mas Belotti marca e garante empate para o Torino, futebol internacional12 junho 2024
Juventus sai na frente em derby de Turim, mas Belotti marca e garante empate para o Torino, futebol internacional12 junho 2024 -
 Stranger Things' breakout Millie Bobby Brown on Season 212 junho 2024
Stranger Things' breakout Millie Bobby Brown on Season 212 junho 2024 -
One Punch Man ↳Dublado: 🇧🇷 1ª - Animes Dublado no Gdrive12 junho 2024
-
 Assistir Boushoku no Berserk - Todos os Episódios12 junho 2024
Assistir Boushoku no Berserk - Todos os Episódios12 junho 2024 -
 Vídeos Educativos para crianças – Compilação12 junho 2024
Vídeos Educativos para crianças – Compilação12 junho 2024 -
 Moyai Emoji Moai Emoji Easter Island Black Comforter for Sale by BunkerBunch12 junho 2024
Moyai Emoji Moai Emoji Easter Island Black Comforter for Sale by BunkerBunch12 junho 2024
